
kinda fundamental question - talk to me about air locks
02GF74 - 11/12/09 at 11:18 AM
how engine do airlocks cause overheating?
surely the water being forced by the pump can squeeze past the air????
mr henderson - 11/12/09 at 11:32 AM
That's a very good question, I will be interested to read the answers.
I love that avatar, BTW
Bluemoon - 11/12/09 at 11:32 AM
Yes, but the water ways split inside the engine, so there is more than one way for the water to go complicating matters... It will take the easy
path.
Also think upside-down U bend water can pass with an air pocket at the top but the flow is restricted, and the air-pocket can't be removed..
i.e. to get it out you would need an air bleed at the top of the bend..
It's probably even worse than that if you think about the air expanding as it gets hot the air in the trapped in an upside down U bend gets
bigger (as the heater tank vents) causing more restriction..
Dan
[Edited on 11/12/09 by Bluemoon]
blakep82 - 11/12/09 at 11:35 AM
if you have an air lock, there will be bits inside the water way which will not be immersed in water and so the heat won't be taken away.
water will pass through it, but only a much smaller amount
02GF74 - 11/12/09 at 12:06 PM
have to admit I am fining it hard to visualise.
let's keep i simple to upside down U e.g. as per thermostat housing or top of radiator.
one of two things happen when water is being pumped:
a) the water pushes the air through the system
b) the water flows "under the air" - so as ^^^ say, a lot less will flow - not sure I can picture that.
mr henderson - 11/12/09 at 12:10 PM
Perhaps it depends where the air lock is. Most of us have topped up the water system in a car, then run the engine for a bit, then had to top it up
again, because the pump has pushed the air around with the water.
blakep82 - 11/12/09 at 12:17 PM
like this
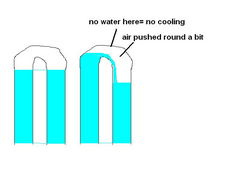
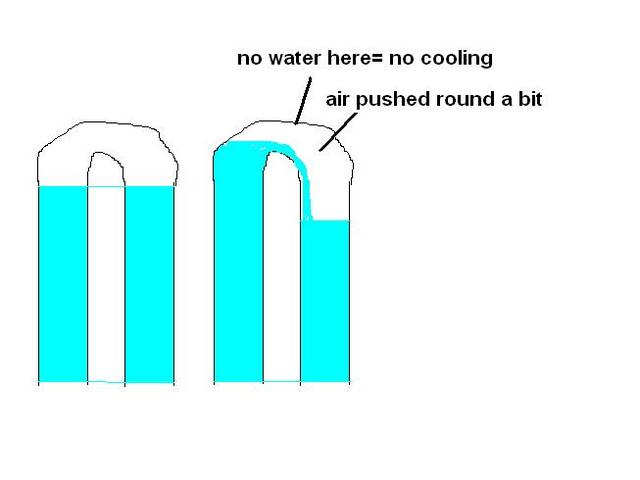
Description
you're right, i didn't mean less will flow, but it won't flow as easy.
normally the pump will be pumping water up as fast as its coming down, gravity will help keep water moving up. and no real effort on the pump
with too much air the pump willbe pumping against gravity also
[Edited on 11/12/09 by blakep82]
blakep82 - 11/12/09 at 12:19 PM
quote:
Originally posted by mr henderson
Perhaps it depends where the air lock is. Most of us have topped up the water system in a car, then run the engine for a bit, then had to top it up
again, because the pump has pushed the air around with the water.
that wouldn't strictly be an air lock though would it? an air lock would be permanantly trapped air i think
craig1410 - 11/12/09 at 12:52 PM
If it helps to visualise then pretend it isn't air, pretend it is a piece of cork or polystyrene instead. If we are talking about a U bend then
the air/cork/polystyrene will stay more or less in position and will restrict the flow capacity of the hose.
I don't think it will tend to expand much when hot, as one of the posters above said, because the system is pressurised and this will counteract
the expansion due to temperature to a large extent. If anything I would expect the air volume to decrease slightly because the expanding water volume
(due to heat) will be more 'powerful' than the expanding air. This is after all why we run pressurised systems, to prevent water turning to
steam.
I hope this helps,
Craig.
stevebubs - 11/12/09 at 12:53 PM
Correct and whilst the pressure of the water will compress the air, it will only compress until the pressure of the 2 liquids (gas is a liquid) is
equal.
Bluemoon - 11/12/09 at 01:03 PM
quote:
Originally posted by craig1410
I don't think it will tend to expand much when hot, as one of the posters above said, because the system is pressurized and this will counteract
the expansion due to temperature to a large extent. If anything I would expect the air volume to decrease slightly because the expanding water volume
(due to heat) will be more 'powerful' than the expanding air. This is after all why we run pressurised systems, to prevent water turning to
steam.
I hope this helps,
Craig.
Yes and no. If there is not to much air, yes. Air will expand to a larger extent than water as it's a gas pressure is proportional to
temperature, if the system was vented.
Thus if the pressure becomes high enough (because of an airlock of sufficient cold volume) venting via the pressure cap occurs allowing the air to
expand.
Having worked this out the temperature increase will be too small, to allow this i.e. temperature increase by ~100C would cause a 1/3rd pressure
increase thinking about it for a gas, that's not enough to open the pressure cap (would require 100% pressure increase i.e. 15psi or so).
Water expansion is probably the dominate effect as the temperature increases..
Thanks Craig that made me think a bit harder about the physics!
So this effect will have little to do with airlocks, if anything the water expansion will be the dominate one then, if anything will help as the
airlock volume will be squashed as the water expands, probably decreasing the effect of an airlock.
Dan
[Edited on 11/12/09 by Bluemoon]
Dusty - 11/12/09 at 01:13 PM
Other effects? Suppose the air is under the thermostat as in a crossflow. Thermostat will not open. Air also replaces some of the water in the system
reducing efficiency. Suppose the air is trapped in the head. Hot spots, poor cooling, localised boiling?
That it does reduce flow is clear from all the old cars you drove that had poor heaters that warmed up when you bled them.
Probably because the pumped water will flow via the line of least resistance. Offered an option of pushing the water column a few inches uphill past
an empty length of hose or downhill through full hose it goes down. In the pic below if you keep uprating the pump you will get to a stage where the
lower loop offers the same resistance to flow as the hydrostatic pressure difference of the columns in the upper loop and flow will then start over
the top. Sort of. Many factors to consider.
Also depends on the power of the pump. I suspect most are only just up to the job.
[Edited on 11/12/09 by Dusty]
Rescued attachment cool.JPG
Steve G - 11/12/09 at 01:17 PM
I'd say fairly simply put, if you have an air lock which will end up at the highest point this will either restrict or block the flow of coolant.
The energy from combustion (ie heat) needs to be dissipated - this is normally done by being transferred to the coolant, and to then be transferred to
heat the air flowing across the radiator.
If the flow is restricted around the cooling system, you lose or restrict the capacity for the heat energy to be removed from the engine and so the
metal will get hotter and hotter (and probably warp). The coolant in contact with this metal getting hotter and hotter will soon turn to steam and so
pressurise the system with fairly obvious consequences. Something will give eventually - be it the engine warping, a pressure cap releasing, or a
coolant hose going pop.
Does that sound logical??
Findlay234 - 11/12/09 at 01:27 PM
With an expansion tank set up the more locked in air you have the less fluid there is in the system to remove heat. Water has a much higher specific
capacity and conductivity than high pressure air.
Any air in the system, whether it stays in situ, partially blocks the fluid flow or even just flows around the system with the water will reduce the
over cooling effect due to the reduced volume of working fluid.
As far as i can work out (in my head) there should be no situation where a bubble of air actually stops water moving round the system. It should act
just like a fluid albiet a less dense fluid so more likely to rise to the top of any U bends especially in low pressure cases such as the engine being
off or where you have very large diameter tubing. Large diameter tubing, where the cross sectional area is far greater than needed for your water
pumps flow rate will allow air to form bubbles in a U bend. This bubble shouldnt stop any water from still flowing around but it will reduce the
capacity to remove heat from the bend itself and by reducing the overall level of coolant fluid (the water) from the system the overall cooling
efficiency will go down. There shouldnt be an issue with this happening within the engine beacuse the internal water ways "should" be
designed to match the pumps flow rate so should push any air out.
If there was a case where the air formed a blockage then as the pump continues to spin the water in front of the air block should gain pressure and
the water behind the air block should loose pressure. This pressure differential will move the blocking fluid 'Air'
Therefore an air bubble should not stop your fluid moving around the system.
I will now say that it depends on the size of the bubble. if you have a bubble so large that it takes up a large percentage of the coolant volume then
the pump may get to a point where it starts to cavitate or even just spin in air. therefor no fluid will be moving around at all. I think this is a
very rare case and nothing to do with bubbles, this is a major loss of coolant fluid.
Oh ive also thought that you if you had a badly designed system that could maintain a large air bubble around the thermostat then this would
'lock' out the rest of the system and no coolant would pass through the radiator. but this shouldnt happen unless your thermostat is at the
top of your system and uses large diameter tubing...
[Edited on 11/12/09 by Findlay234]
02GF74 - 11/12/09 at 01:51 PM
quote:
Originally posted by craig1410
If it helps to visualise then pretend it isn't air, pretend it is a piece of cork or polystyrene instead. If we are talking about a U bend then
the air/cork/polystyrene will stay more or less in position and will restrict the flow capacity of the hose.
I hope this helps,
hmmmm, I think I may have cracked it - I wasn't thinkg of the air being under pressure.
refering to the U-bend above.
With engine from cold, water will be pumped under the "lump" of air without any problems but as the system is sealed, the water will expand
thus increasing the pressure within the system.
As the air becomes under higher pressure, it stays at the highest point being less dense but the pump will find it harder to pump the water past
it.
My analogy.
Take a gulp of water, stick a toilet inner tube over yer gob with your hand over the other end. Now try to spit the water out. I reckon your hand
will get wet.
Now do the same but this time put the air in the tube under pressure. Unless you have the lungs of Geoff Capes, you are unlikley to squirt much, if
any, water out yer gob.
one more thing.
but you could say what if the air was water, then how can the pump ever work?
I think the difference here is that the water would be same density so is not resisting flow as the mnuch less dense air. If the pump was powerful
enough (or the tube small enough) , it would push the air round the system. maybe?
It would be interesting to see this happen with a transparent tube.
[Edited on 11/12/09 by 02GF74]
Findlay234 - 11/12/09 at 02:27 PM
quote:
Originally posted by 02GF74
quote:
Originally posted by craig1410
If it helps to visualise then pretend it isn't air, pretend it is a piece of cork or polystyrene instead. If we are talking about a U bend then
the air/cork/polystyrene will stay more or less in position and will restrict the flow capacity of the hose.
I hope this helps,
hmmmm, I think I may have cracked it - I wasn't thinkg of the air being under pressure.
refering to the U-bend above.
With engine from cold, water will be pumped under the "lump" of air without any problems but as the system is sealed, the water will expand
thus increasing the pressure within the system.
As the air becomes under higher pressure, it stays at the highest point being less dense but the pump will find it harder to pump the water past
it.
My analogy.
Take a gulp of water, stick a toilet inner tube over yer gob with your hand over the other end. Now try to spit the water out. I reckon your hand
will get wet.
Now do the same but this time put the air in the tube under pressure. Unless you have the lungs of Geoff Capes, you are unlikley to squirt much, if
any, water out yer gob.
one more thing.
but you could say what if the air was water, then how can the pump ever work?
I think the difference here is that the water would be same density so is not resisting flow as the mnuch less dense air. If the pump was powerful
enough (or the tube small enough) , it would push the air round the system. maybe?
It would be interesting to see this happen with a transparent tube.
[Edited on 11/12/09 by 02GF74]
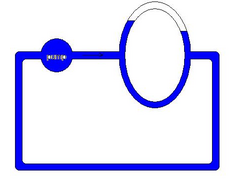
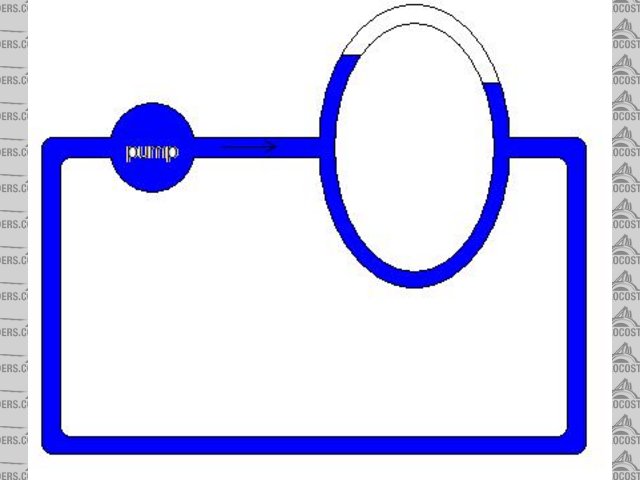
No this is not a good analogy, your hand is the blockage in your system not the air. the cooling system is also a closed loop system so better to
think in these terms... attached image...
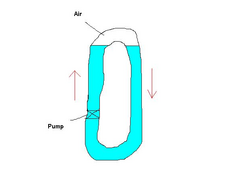
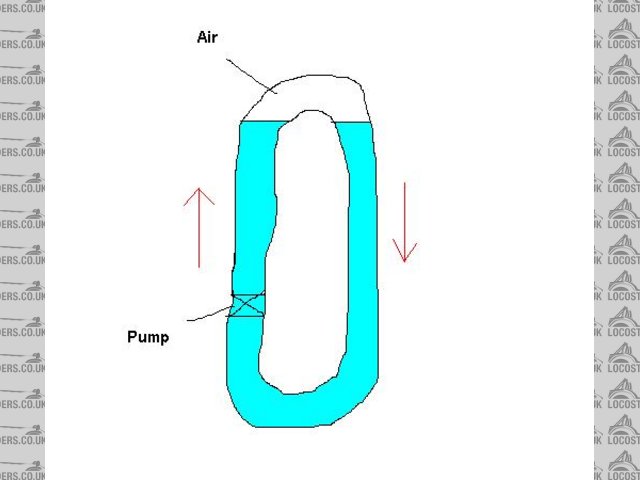
Rescued attachment untitled1.JPG
Findlay234 - 11/12/09 at 02:30 PM
The fluid will continue to move around regardless of the air in the system (to a point where the air takes over most of the system and the pump starts
to cavitate)
The cork analogy is also wrong because we are talking about a fluid here not a solid. ok it is a fluid under pressure but even a very viscous fluid
under high pressure (maybe use oil in a shock absorber) doesnt act like a cork.
In a closed loop system and as long as there are no 'solid' blockages to the loop then at any pressure the pump stength is not important.
you could have a computer fan in there (as long as the casing and components can handle the pressure) then it will be able to pump the fluid
around.
If the pressure in the system is at 5Bar then that doesnt mean the pump is working against 5bar because at rest the pressure behind the pump is also
at 5bar. the pump creates the pressure differential for the the fluid to flow. for a given fluid flow rate the pump only needs to be strong enough to
overcome the resistance to flow which will only come from the friction (including blockages). The viscosity of the fluid changes the level of friction
and although its dependant on pressure the difference is very small.
[Edited on 11/12/09 by Findlay234]
02GF74 - 11/12/09 at 02:45 PM
well I am back to square one - as ^^^^ , the water will be pumped around.
so if air lock is in thermostat hoiusing (e.g. crossflow, so what, water is still getting round.
or in top of radiatr - so the top 1 or 2 cm see air but water is flowing thoruugh the vast majority of the radiator.
hence how is the air lock bad?
getting more confused ... 
Findlay234 - 11/12/09 at 02:46 PM
This is all from memory by the way people so I stand to be corrected.. 

Cheers
Steve G - 11/12/09 at 02:53 PM
Water may well be pumped around - but the flow will be restricted to a greater or lesser degree by the air bubble. Think of a pipe in the shape of an
upturned "U" containing an air bubble and try blowing the water through. It'll take more effort than if there were no bubble. The water
pump can only give a certain amount of effort.
02GF74 - 11/12/09 at 03:00 PM
quote:
Originally posted by Steve G
Think of a pipe in the shape of an upturned "U" containing an air bubble and try blowing the water through. It'll take more effort than
if there were no bubble.
I'm trying but nothing's happening. 
Why?
Findlay234 - 11/12/09 at 03:02 PM
An air bubble that occurs in your thermostat (and doesnt move) and stops (or locks) it is bad because then the cooling fluid will only move around the
internal waterways and not through the radiator. thereby limiting the volume of cooling fluid and limiting the surface area for heat exchange to the
atmosphere (something the radiator does very well)
If you have an air bubble in the radiator then you again limit the volume of fluid in the system and it can be hard to remove because the total cross
section of the radiator can be larger than the waterways to and from it and larger than the pumps capacity therefore creating a nice low pressure area
for the bubble to congregate. The bubble also has the effect of blanking off a portion of the radiator... not good. I think off the top of my head its
best to either have an air bleed at the top of the rad or to have the inlet to the rad at the bottom and the outlet at the top so the air flows with
the fluid as best it can...
A non moving air bubble in the pump is bad because you pump will cavitate and as its not an air pump will become useless. this would have to be a very
badly designed system with the pump at the top or a system that had run very dry.
Air bubbles in the rest of the system will lower the volume of working fluid so will make the system less efficient but shouldnt stop it working..
The only time i think i could ever see an air bubble stopping fluid flow (apart from the cavitation mentioned) would be a very tall system where the
pumps strength was unable to overcome the pressure created by the weight of the water above it. This would have to assume that there was more fluid on
the high side of the system and would factor in gravity.. I doubt any of our systems are too weak to overcome the changes in potential energy seen.
Findlay234 - 11/12/09 at 03:06 PM
Where are you having a problem??
02GF74 - 11/12/09 at 03:10 PM
the upside down u-bend.
if I take my garden hose, and hold it so that there is an upside down u-bend, even at lower pressure, I reckon water is gonna flow.
I am not getting how the air at the top of the U is stopping the water from flowing.
Findlay234 - 11/12/09 at 03:24 PM
where are you talking about? in a toilet? in your car cooling system?
Do you actually have an issue where an 'air lock' is actually restricting flow or are you trying to hypothetically understand it?
Cheers
Fin
Bluemoon - 11/12/09 at 03:41 PM
quote:
Originally posted by blakep82
like this


Description
you're right, i didn't mean less will flow, but it won't flow as easy.
normally the pump will be pumping water up as fast as its coming down, gravity will help keep water moving up. and no real effort on the pump
with too much air the pump willbe pumping against gravity also
[Edited on 11/12/09 by blakep82]
The above picture has it in one, the only time water would not flow is when the pump can't lift it higher than the U. i.e. imagine the pump has
to lift the water from the level condition, to the one where it's lifted up high enough to "fall" over the U that requires the pump to
build up pressure. Thus a pump will have some upper pressure that it can't exceed so the water would stop flowing with a sufficient height.
I guess any extra pressure requirement from this air gap probably also slows the flow (as the pump has to work to move the water as the bubble of air
is there). Thus reducing the cooling efficiency. Without the air in the U no pressure difference would be needed to create the flow and the pump will
flow the largest amount fluid it can.
Like others have said an airlock in the thermostat housing is bad news, as it will never open as the thermostat not warm up.. You would know this is
happening though as the rad would not get warm..
Dan
[Edited on 11/12/09 by Bluemoon]
Steve G - 11/12/09 at 03:50 PM
The air bubble always rises to the highest point. Blow against it and you are blowing against that bouyancy for want of a better word - you are trying
to force the bubble through the tube and it naturally wants to return to the highest point
02GF74 - 11/12/09 at 04:47 PM
quote:
Originally posted by Findlay234
Do you actually have an issue where an 'air lock' is actually restricting flow or are you trying to hypothetically understand it?
no and yes.
let's say it is crossflow thermostat housiing, and we can approximate this to the u-bend above.
let's also say that there is ari above the termostat and that there is no small hole in the thermostat.
in normal situation, there is water above hte thermostat.
engine warms up, the water below the thermostat in which the valve is immersed opens and water flows to the radiator.
now let's say the is air above the thermostat.
how is it going to be different.
like before, no water flows due to thermostat being closed. as before once the wax gets hot enough the valve will open. now the situation is
different as there is air above the thermostat.
I cannot believe that water will not flow past the air.
convince me otherwise.
Bluemoon - 11/12/09 at 05:50 PM
Arrr the thermostat is in the airlock, it has not warm water around it only air.. Then it will probably not open as the thermostat is cooled by the
surrounding metal, the conduction of air is poor compared with water (other wise we would use air to cool the engine rather than water)..
This is why the hole (or bypass flow in some cases) is important, as lets the air out and ensure the thermostat is always submerged (particularly on
the hot side)... The hole also allows a small flow of hot engine coolant to ensure the thermostat is around the same temperature of the engine..
Dan
[Edited on 11/12/09 by Bluemoon]
Nash - 11/12/09 at 06:22 PM
OK think of it like this:
At Cold start up in the Thormostat housing the stat is closed. The water warms up and opens the stat and the water flows. The stat opens because the
water is getting hotter and hotter due to it NOT flowing. It doesn't open due to pressure build up. OK so far?
Now in the case of the Air Lock the flow is restricted or completely blocked. If I read you correctly you think the pressure will build and eventually
move the air? Well two things prevent that:
1. The water will take the path of least resistance and water will move elsewhere not allowing pressure build up
2. The pump will not build pressure as its not a positive displacement pump. Its a constant velocity pump so the water just circulates around the
gears (it takes the path of least resistance around the pump casing)
So the pressure never increases and the air isn't forced to move. The flow stalls and the engine overheats.
Hope that helps?
..........Neil
[Edited on 11/12/09 by Nash]
jollygreengiant - 11/12/09 at 06:33 PM
The problem here is that you think in two dimension instead of three. You are thinking purely in terms of water OR air. With a cooling sytem ther is a
third dimension and that is of the the hot intermediate state of water, STEAM.
Once you have an air lock develop, then you have a surface which is NOT cooled. This surface then gets very hot and any water that makes contact with
the uncooled areas perifery will almost certainly turn to steam thus increasing volume and thereby uncooled area of engine internals.
What annoys me about some manufacturers is that if you look closely at some cooling systems then they expect water and air not follow the laws of
physics, some designers expect air to flow down through water when you look at the hose arrangement, (some rovers spring to mind)
Toltec - 11/12/09 at 07:58 PM
Interesting thread.
Thinking about how air could restrict flow -
Archimedes principle tells us that the upthrust generated by the air is equal to the mass of the volume of water it displaces. The pump is trying to
move all of the contents of the system, the air effectively pushes back against the pump.
Imagine a column of water mixed with air where the water flow is downwards and just sufficient to keep the air bubbles circulating in an approximately
static region. At any particular point in the pipe the area of the pipe available for fluid flow is reduced by the cross sectional area of all of the
bubbles at that point.
Fluid dynamics is obviously rather more complex and there will be further losses, however just taking the two above it is easy enough to see that air
will cause a loss of flow.
The other thing I did not notice any mention is that the pressure in the system is not even throughout it. The pressure in the head/block is much
higher than the radiator etc. There are flow restrictors at the water exits from the head that help keep the pressure much higher to prevent boiling
around the exhaust ports. Not sure what difference this makes, I suspect the higher pressure would make air bubbles easier to shift since they should
be smaller.
hoots_min - 11/12/09 at 08:05 PM
Here's my tuppence.
Water will naturally contain a certain amount of air which will vary dependent upon temperature - the hotter the water, the more air will be contained
within it. This is why you will have more air present int the system when it is cold and why it is better to bleed the air out when it is cold.
Having air in your system be it pressurised or non-pressurised will ultimately result in a reduction of cross sectional area available for flow.
Taking the upside down U bend as example, the pressure at both sides of the air bubble will be virtually equal, the only difference being that the
upstream side will be ever so slightly higher to overcome the frictional pressure drop of the water through the pipeline. As a result the bubble may
move ever so slightly forward but the pressure difference will be very small (we are talkin millibars here as there are only very short distances
involved). The air bubble will therefore not move under steady state conditions (i.e. stable flow).
As mentioned above also, the presence of the air will result in a drop off in flow. This is a result of the cross sectional area (CSA) that is taken
up by the air bubble. Think of it as a natural restriction, i.e. 1" pipe then going into a ½" pipe and then back to a 1" pipe.
Reducing the CSA by half actually increases the pressure drop in that section by 32!! (the DP is related to 1/dia^5). So you therefore get a drop off
in flow because of the presence of the air; if the bubble is present in a section downstream of a split, then flow will preferrentially favour the
path of least resistance.
Under steady flow, the bubble will not move. However, if you are able to create a differential across the bubble, for example create a vacuum or a
large pressure on one side, the bubble will move. The 'incompressible' nature of water means it can produce a 'water hammer'
effect when a pump is started up which may push the bubble along if it is close enough to the pump, but after the tortuous route the water generally
has to go through, the energy from the pressure wave will likely have dissipated.
(I write 'incompressible' in apostrophes as there may be some out there who argue that water is compressible. To a degree it is and
compressibility factors are readily published, however, at the pressures we are discussing here, it can be regarded as incompressible.)
hope this helps.


